北京百普赛斯生物科技股份有限公司品牌商
14 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
靶向BCMA免疫疗法:推动多发性骨髓瘤治疗进入新纪元
1473 人阅读发布时间:2020-10-12 17:06
B细胞成熟抗原(B-cell maturation antigen, BCMA),又称TNFR超家族成员17(TNF receptor superfamily member 17,TNFRSF 17),与家族成员TACI和BAFF-R可分别与BAFF或APRIL两种配体相结合,这三种受体和两种配体共同组成的系统可以对免疫活性细胞进行多方面的调控[1] (图1)。BCMA主要在浆细胞和成熟B淋巴细胞中表达,在其它的正常组织上基本无表达,相关研究表明BCMA是多发性骨髓瘤细胞系上最具选择性表达的受体,且表达量随着疾病的发展而逐步递增[2],因此,BCMA成为治疗多发性骨髓瘤的理想靶点。

图1. The A-proliferation inducing ligand/B cell maturation antigen (APRIL/BCMA) pathway and its biological effect in the multiple myeloma bone marrow microenvironment
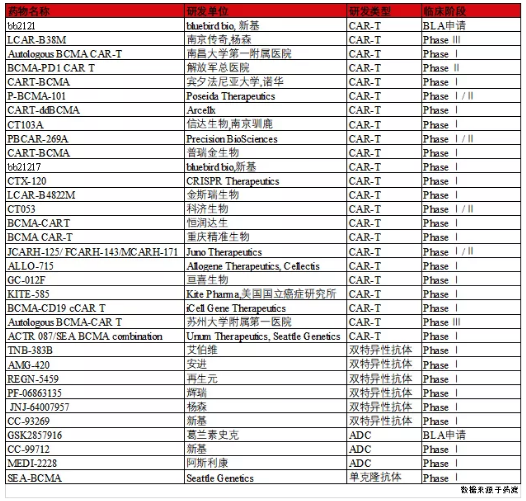
根据药渡数据库显示,目前靶向BCMA的免疫疗法主要包括嵌合抗原受体T细胞疗法(CAR-T)、抗体药物耦联物(ADC)和双特异性抗体三大类(表1)。其中在ADC疗法中表现突出的是葛兰素史克的GSK2857916,目前正在接受FDA的优先审查和欧盟EMA的加速评估,而以安进AMG-420和艾伯维TNB-383B为代表的双特异性抗体药物的研究基本处于临床早期阶段;另外关于BCMA CAR-T细胞领域的开发角逐十分激烈,国内外多家医药企业均有相关研发管线的布局,其中蓝鸟生物bb2121的临床数据显示,患者经bb2121治疗后客观缓解率(ORR)为73.4%,完全缓解率(CR)为31.3%,中位随访时间为11.3个月,无进展生存期(PFS)达到8.6个月[3]。紧随其后是南京传奇的LCAR-B38M,公开数据显示,中位随访时间为6个月,总缓解率达100%,完全缓解率达86%[4]。目前bb2121和LCAR-B38M两款产品均处于临近上市阶段,虽然蓝鸟生物的bb2121在提交BLA申请后被FDA拒绝,但该药物在治疗多发性骨髓瘤的临床试验中已经取得了不错的疗效,另外随着南京传奇在纳斯达克的上市或许能够加速LCAR-B38M的申报流程。综上,随着对BCMA靶点研究的不断深入,以BCMA为靶点的抗体药物和CAR-T细胞疗法必定会为多发性骨髓瘤患者带来新的曙光。
表1. Summary clinical study results of targeting BCMA
ACROBiosystems作为专注于生物药研发领域的蛋白供应商紧跟研发前沿,开发了一系列不同种属和标签的高生物活性BCMA蛋白,通过Flow cytometry、SPR、ELISA等检测方法对其性能进行了全方位的分析,适用于免疫、抗体药筛选、药代动力学(PK)研究及Anti-BCMA CAR表达检测,更有荧光标记和生物素标记的BCMA蛋白加速您的研发进程。
产品数据
>>> 案例1 流式细胞术检测Anti- BCMA CAR阳性表达率
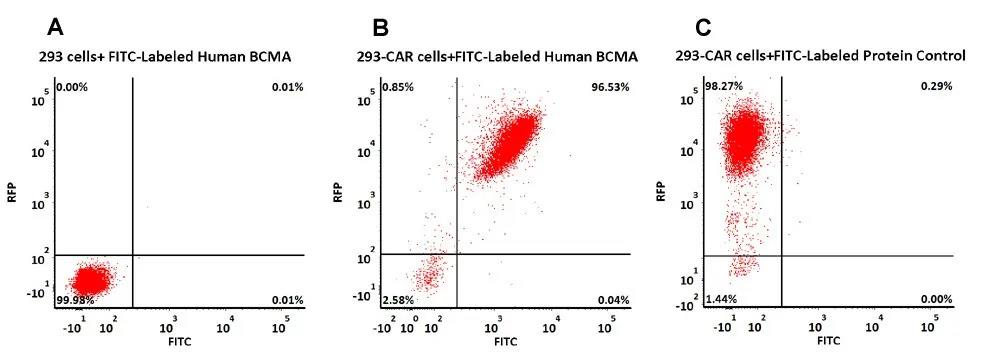
293 cells were transfected with anti-BCMA-scFv and RFP tag. 2e5 of the cells were stained with FITC-Labeled Human BCMA, His Tag (Cat. No. BCA-HF2H1, 1 µg/ml) and FITC-labeled protein control. Non-transfected 293 cells and FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-BCMA-scFv) expression and FITC was used to evaluate the binding activity of FITC-Labeled Human BCMA, His Tag (Cat. No. BCA-HF2H1) (QC tested).
>>> 案例2 ELISA检测CD3 X BCMA双特异性抗体
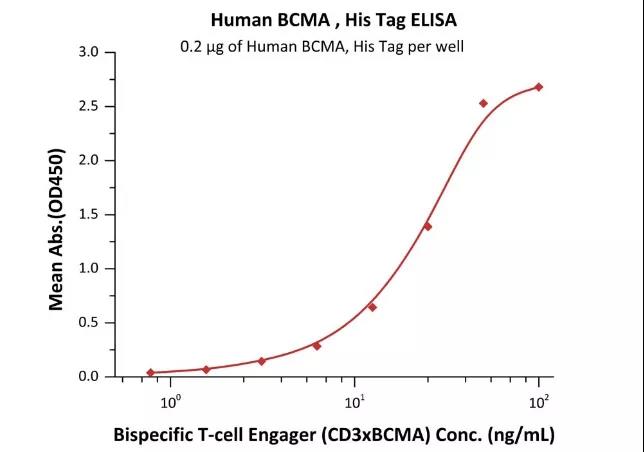
Immobilized Human BCMA, His Tag (Cat. No. BCA-H522y) at 2 μg/mL, add increasing concentrations of Bispecific T cell Engager (CD3 X BCMA) in 10% human serum and then add Biotinylated Human CD3E&CD3D Heterodimer Protein, His,Avitag&Tag Free (Cat. No. CDD-H82W6) at 0.2 μg/mL. Detection was performed using HRP-conjugated streptavidin with sensitivity of 15 ng/mL (Routinely tested).
>>> 案例3 基于ELISA的中和抗体筛选试验
抗BCMA中和抗体能有效阻断BAFF和BCMA的结合,IC50=4.877μg/mL
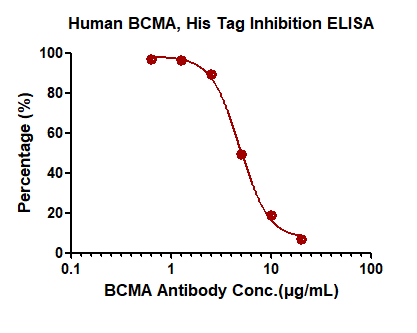
Immobilized Human BCMA, His Tag (Cat. No. BCA-H522y) at 2 μg/mL (100 μL/well) can bind pre-mixed increasing concentrations of Anti-BCMA Neutralizing Antibody, Mouse IgG1 (Clone: AM43, Cat. No. BCA-M43) and 0.01 μg/mL (100 μL/well) Biotinylated Human BAFF, His,Avitag (Cat. No.BAF-H82Q2) with a half maximal inhibitory concentration (IC50) of 4.877 μg/mL (Routinely tested).
抗BCMA中和抗体能有效阻断APRIL和BCMA的结合,IC50=4.624 μg/mL
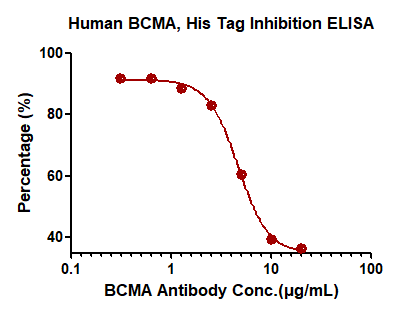
Immobilized Human BCMA, His Tag (Cat. No. BCA-H522y) at 2 μg/mL (100 μL/well) can bind pre-mixed increasing concentrations of Anti-BCMA Neutralizing Antibody, Mouse IgG1 (Clone: AM43, Cat. No. BCA-M43) and 0.05 μg/mL (100 μL/well) Biotinylated Human APRIL, Fc,Avitag (Cat. No.APL-H82F5) with a half maximal inhibitory concentration (IC50) of 4.624 μg/mL (Routinely tested).
产品列表
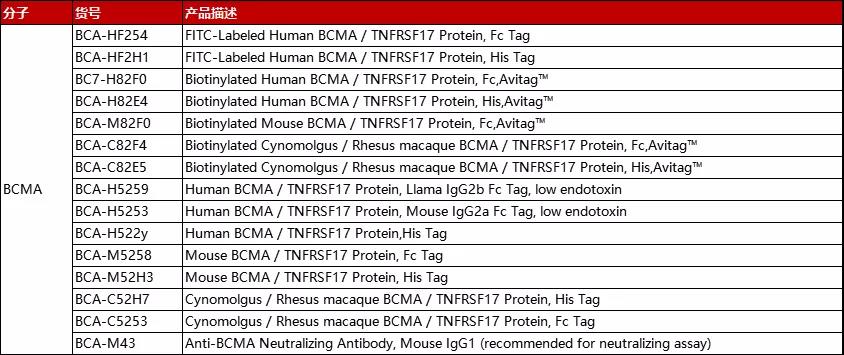
分子:BCMA
(点击图片查看产品详情)
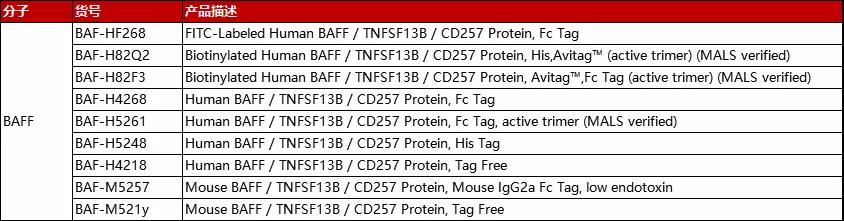
分子:BAFF
(点击图片查看产品详情)
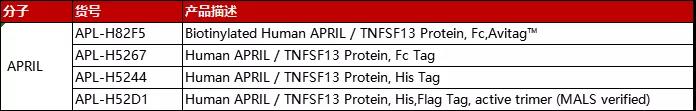
分子:APRIL
(点击图片查看产品详情)
您可通过以下方式联系到ACROBiosystems:
邮件:order.cn@acrobiosystems.com
电话:13521050293
微信:扫描下方二维码即可进行沟通并可邀请加入生物药研发资源分享群

(请备注公司+姓名)
参考文献
[1] Cho SF, Lin L, Xing L, et al. BCMA-Targeting Therapy: Driving a New Era of Immunotherapy in Multiple Myeloma.[J]. Cancers (Basel). 2020, 12(6):E1473.
[2] Carpenter R O, Evbuomwan M O, Pittaluga S, et al. B-cell Maturation Antigen Is a Promising Target for Adoptive T-cell Therapy of Multiple Myeloma[J]. Clinical Cancer Research, 2013, 19(8):2048-2060.
[3] Bristol-Myers Squibb and bluebird bio Announce Positive Top-line Results from the Pivotal Phase 2 KarMMa Study of Ide-cel in Relapsed and Refractory Multiple Myeloma.
[4] https://www.genscript.com.cn