北京百普赛斯生物科技股份有限公司品牌商
14 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
【免费试用装】T细胞激活最佳搭档 —— CD3/CD28抗体偶联磁珠
1224 人阅读发布时间:2022-08-25 13:04
T淋巴细胞如何被激活?
在机体内,T淋巴细胞的活化,在免疫应答中扮演着相当重要的角色,研究表明,诱导T细胞的活化与增殖需要两种信号:第一信号是TCR/CD3与抗原提呈细胞(APCs)表面特异的MHC分子抗原肽复合物结合产生的特异性抗原刺激信号;第二信号是非特异性的共刺激信号,由APCs表面多对共刺激分子和T细胞的相应受体相互作用后产生(如:>CTLA-4和CD80、CD86, 4-1BB和4-1BBL,CD40和CD40L,PD-1和PD-L1等),其中CD28是最为重要的共刺激分子,第二信号可使T细胞完全活化,分泌细胞因子和表达细胞因子受体[1], 如果缺乏共刺激信号,第一信号非但不能有效激活特异性T细胞,反而导致T细胞失能[2];而在体外,联合使用CD3、CD28的抗体刺激T细胞,模拟体内T细胞活化的双信号作用,是目前体外进行T细胞激活与扩增应用最广泛的方法[3]。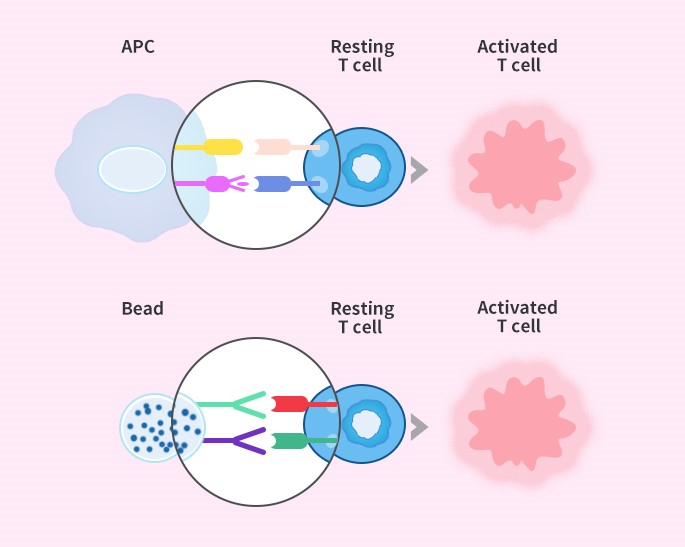
细胞治疗领域中T细胞的激活
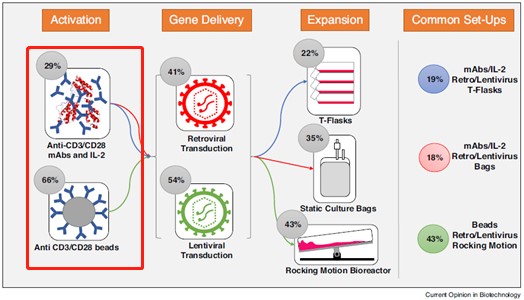

★ 5.5 μm大小,可更好的模拟APC,刺激T细胞
★ 磁性强,轻松分离,磁珠不易残留
★ 超低内毒素(< 2EU/mg),对T细胞无伤害
★ 经细胞水平验证,可高效激活扩增T细胞

产品数据
☛ Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001) 可高效激活T细胞
(添加文末小助手咨询更多)
☛ 经Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001) 刺激后,可对T细胞进行有效扩增
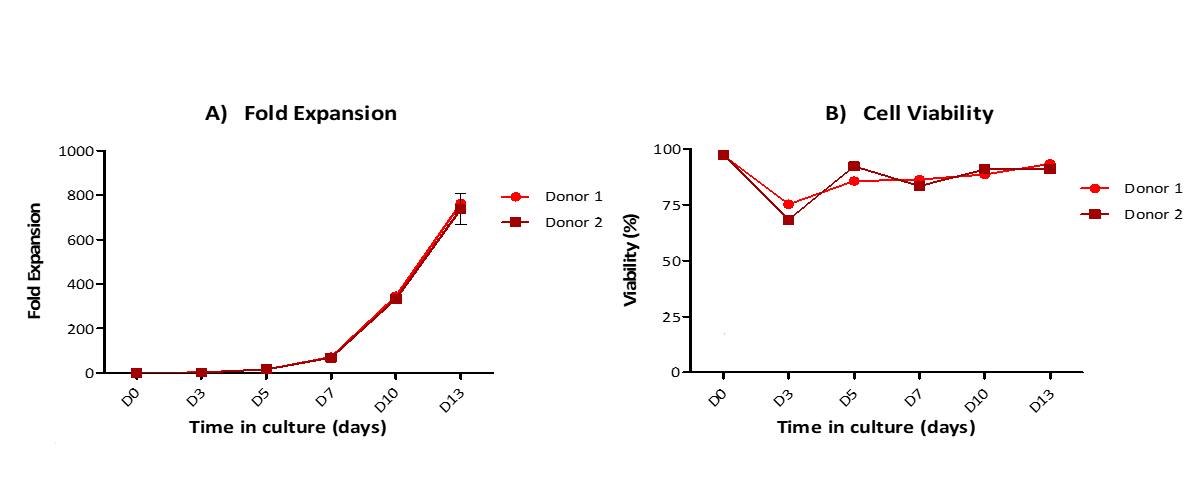
(添加文末小助手咨询更多)
竞品对比数据
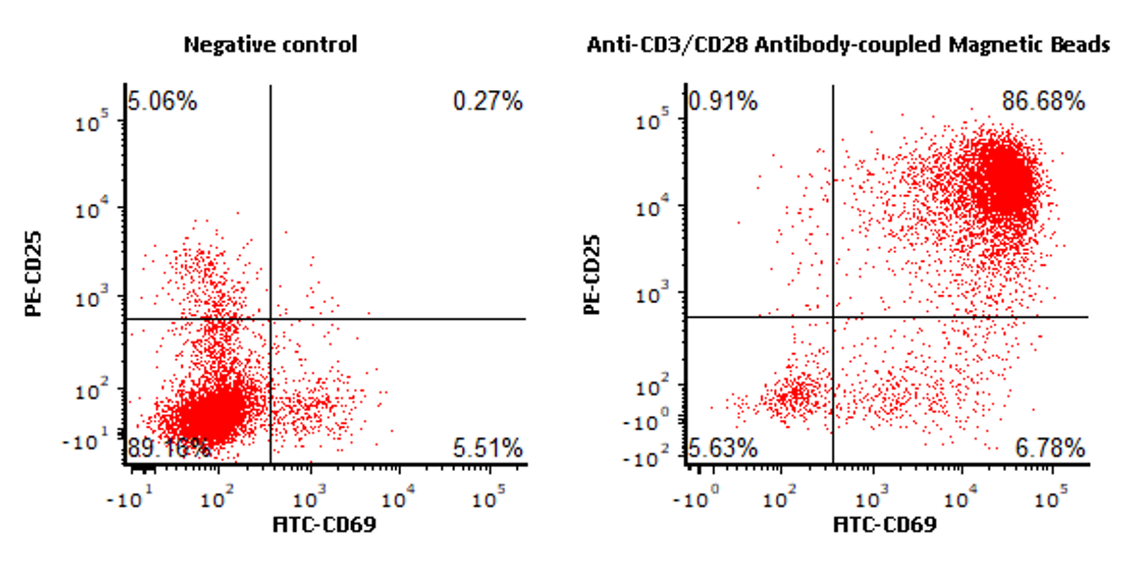
ACRO Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001) 与竞品分别激活T细胞,激活能力水平基本一致
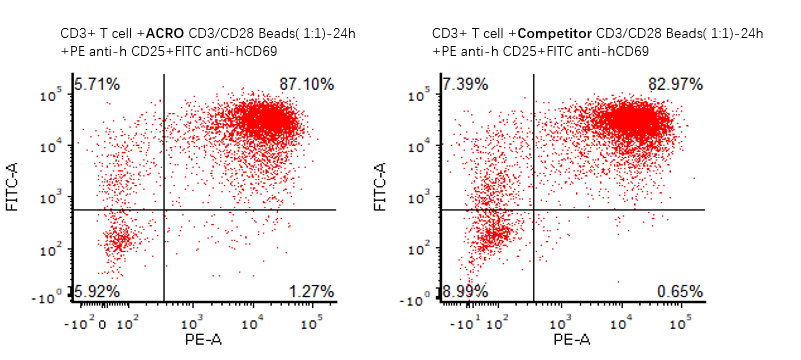
The purified human T cells were activated using ACRO Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001) and competitor’s beads respectively at a ratio of 1:1 beads-to-cells for 24 hours with RPMI1640 supplemented with 10% of FBS. Cells were fluorescently stained using PE labeled anti-human CD25 antibody and labeled FITC anti-human CD69 antibody and analyzed by flow cytometry.
(添加文末小助手咨询更多)
与竞品相比,ACRO Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001),可对T细胞进行有效扩增,且有较高水平的扩增能力
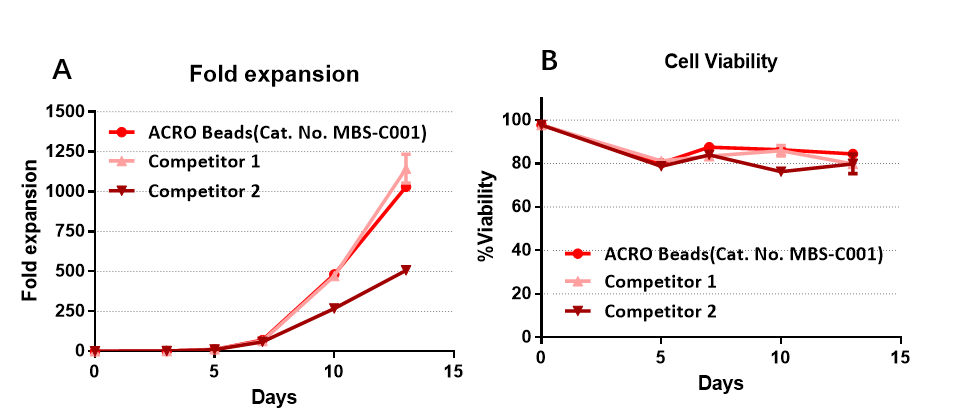
The purified human T cells were stimulated using Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001) and competitor’s beads respectively. Cells were expanded in T cell culture medium supplemented with 4ng/mL of rhIL-2 Protein (Acrobiosystems, Cat. No. IL2-H4113). Activated Cells were expanded for up to 13 days (A) with high cell viability (B).
(添加文末小助手咨询更多)
★添加文末小助手了解更多T细胞激活试剂Anti-CD3 antibody(clone: OKT3Anti-CD28 antibod
GMP级别细胞因子


ACROBiosystems
inquiry@acrobiosystems.com
微信号:15117918562
(备注:姓名+公司)
参考文献
[1] Kay, J.E., 1991. Mechanisms of T lymphocyteactivation. Immunology Letters 29, 51 – 54
[2] 医学免疫学-第7版
[3] Trickett A, Kwan YL. T cellstimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003Apr 1;275(1-2):251-5.
[4] Kalos M, Levine BL, Porter DL, KatzS, Grupp SA, Bagg A, June CH: T cells with chimeric antigen receptors havepotent antitumor effects and can establish memory in patients with advancedleukemia. Sci Transl Med 2011, 3:95ra73.
[5] Hollyman D, Stefanski J, PrzybylowskiM, Bartido S, Borquez- Ojeada O, Taylor C, Yeh R, Capacio V, Olszewska M, HoseyJ et al.: Manufacturing validation of biologically functional T cells targetedto CD19 antigen for autologous adoptive cell therapy. J Immunother 2009, 32:169-180.
[6]Casati A, Varghaei-Nahvi A, FeldmanSA, Assenmacher M, Rosenberg SA, Dudley ME, Scheffold A: Clinical-scaleselection and viral transduction of human naı¨ve and central memory CD8+ T cells for adoptive cell therapy ofcancer patients. Cancer Immunol Immunother 2013, 62:1563-1573.
[7] Barrett DM, Singh N, Liu X, JiangS, June CH, Grupp SA, Zhao Y: Relation of clinical culture method to T-cellmemory status and efficacy in xenograft models of adoptive immunotherapy. Cytotherapy2014, 16:619-630.



